Batteries are often advertised with a nominal voltage and nominal charge. It’s tempting to take those numbers at face value and assume that regardless of operating circumstances, a battery will sit at its nominal voltage and run until its nominal charge has been depleted. Reality is more complicated than that.
Christopher Milner reminded me of this in a brief conversation this week. He is using 13 3.2V, 100Ah LiFePo4 batteries in series to power the deck motor on his mowing rig. Stringing them together in series gets you close to 48V in their charged state. He reports getting about 3 hours of runtime while powering a motor that consumes 1300W.
This surprises me because my own calculations suggested four 12V, 35Ah lead acid batteries should be sufficient. Christopher obviously knows what he is doing and I trust his experimental results much more than I do my own back of the envelope calculations. The discrepancy means I need to reevaluate my numbers.
When making my calculations I did not take battery discharge rates into account. This is a big mistake, as I’ll show below. But first, for my own educational benefit, I’d like to introduce you to the C-rate, a way to quantify how fast you pull current out of a battery.
The C-Rate
What is a battery’s C-rate? Our good friends at Wikipedia define it as:
The C-rate is defined as the charge or discharge current divided by the battery’s capacity to store an electrical charge.
In layman’s terms, the C-rate is just the ratio of an arbitrary discharge rate to the battery’s charge capacity. It’s a simple way to describe how much current you’re demanding from a battery relative to the battery’s total stored charge. If you’ve got a battery rated for 10Ah and you discharge it at 5A, the C-rate would be:

It’s kind of a janky way of defining things in my opinion. A C is really an inverse hour, or stated differently, the unit of measure for a C-rate is h−1. Also, don’t confuse the C-rate with Coulombs, the unit of measure for charge. Clear as mud?
The reason I write all of this is so we’re all on the same page about what a C-rate is. If I’m wrong, please comment below, because I am going to proceed with this understanding of the C-rate going forward.
The C-rate is a useful measure because the total amount of charge you can extract from a battery depends on how fast you take it out. If you try to pull 100A out of a 10Ah battery, you’re not going to get nearly as much charge out of the battery as you would if you discharged it at 1A.
Additionally, when you demand a large amount of current from a battery, your voltage starts dropping fast. This is important because electrical power, which is ultimately what we’re after, is voltage times current. If your voltage drops you get less power, even if you’re withdrawing the same amount of current from the battery.
Your batteries are going to be much happier and live much longer if you stick with a reasonable C-rate. Having said all of that, how do the four 12V, 35Ah batteries I selected stack up against the expected current draw they’ll experience?
Our SLA Batteries Reevaluated
Previously I estimated the total current consumption of our robot at 197A. I think this is a conservative number, but I’m going to roll with it anyway. The number reflects current consumed by the three deck motors, two drive motors, and various electronics at their worst case scenarios.
I’m using two sets of two 12V, 35Ah batteries wired in parallel, then in series to get an equivalent battery that’s 24V, 70Ah. The discharge rate for one battery in this configuration is half the total current consumption because we have two sets of two batteries wired in parallel. This means that one battery is discharged at a rate of 98.5A.
The datasheet for one of these batteries shows the following chart, with various C-rates:
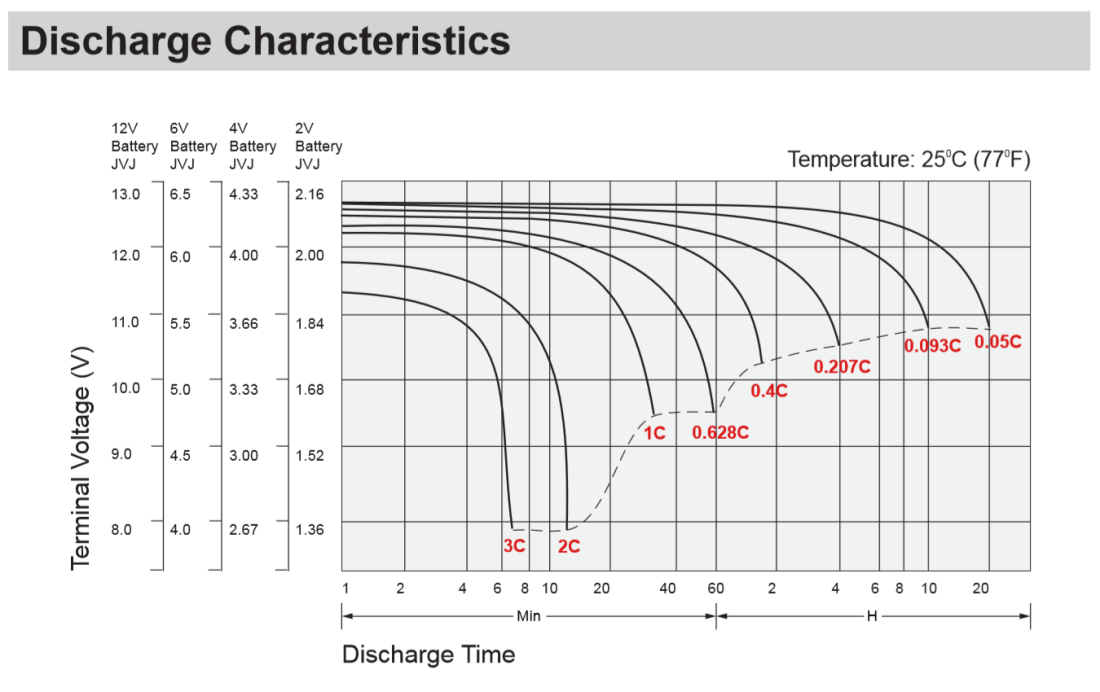
At a discharge rate of 98.5A, our C-rate is 2.81C. Looking at the chart above, that would mean our batteries will last for ~8 minutes. Yikes. And realistically, the 2.81C curve may extend to about 8 minutes, but the voltage drops off so fast after about 4 minutes that you’ll probably start noticing performance problems very quickly.
Also interesting to note from the chart above is that the one hour discharge rate is in fact not 35A as you’d expect with a 35Ah battery. It’s actually 0.628 times 35Ah, or 21.98Ah. To truly extract the 35Ah charge from the battery, you can only discharge it at a C-rate of 0.05C, or 1.75A.
Having written all of this, I wonder to myself why manufacturers don’t just list curves with specific discharge rates in Amps. The chart above would be completely unambiguous if they just showed a curve for 105A, 70A, 35A, 21.98A, etc. The conversion is tedious and honestly, if you don’t truly understand C-rates you leave with the impression that these SLA batteries are much more capable than they truly are.
Looks like we will need to use some lithium batteries after all. Thanks for saving me $400 on some batteries that wouldn’t have worked Christopher!